Water is one of the most important substances on Earth, and it comes in various forms that play crucial roles in our lives. In this chapter, we’ll learn about the different forms of water, how water moves through nature, its properties, and its importance. We’ll also discover how to store drinking water properly.
Forms of Water
Water is incredibly versatile and can exist in three different forms: solid, liquid, and gaseous. Let’s look at each form and understand its characteristics.
Solid Form – Ice
– Description: When water gets very cold, it turns into ice. Ice is the solid form of water.
– Characteristics:
– Temperature: Ice forms at 0°C (32°F) or below.
– Appearance: It appears as solid, frozen crystals.
– Example: Ice cubes, snow, and glaciers.
– Uses: Ice is used to keep things cold, like in refrigerators and coolers. It also plays a crucial role in nature, such as in glaciers and polar ice caps, which help regulate the Earth’s temperature.
Liquid Form – Water
– Description: Water in its liquid form is what we most commonly encounter. It is the form of water that flows and takes the shape of its container.
– Characteristics:
– Temperature: Water is typically found in liquid form between 0°C (32°F) and 100°C (212°F).
– Appearance: It is clear and can be seen in lakes, rivers, and oceans.
– Example: Drinking water, rain, and rivers.
– Uses: Liquid water is essential for drinking, cooking, cleaning, and many industrial processes. It is also vital for all forms of life on Earth.
Gaseous Form – Water Vapor
– Description: When water is heated, it turns into water vapor, which is the gaseous form of water.
– Characteristics:
– Temperature: Water vapor forms when water reaches 100°C (212°F) or higher.
– Appearance: It is invisible, but you can see its effects, such as steam rising from a hot cup of tea.
– Example: Steam from boiling water, fog, and clouds.
– Uses: Water vapor is crucial in weather patterns and the water cycle. It also helps regulate temperature and maintain moisture in the air.
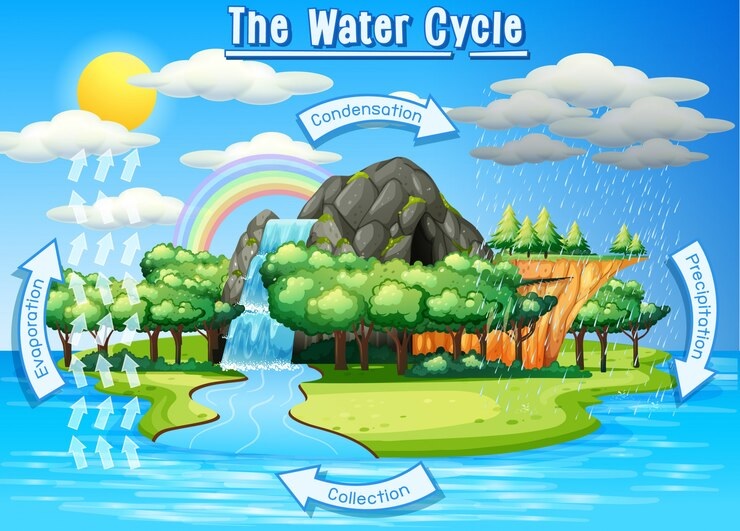
The Water Cycle
The water cycle is a continuous movement of water on, above, and below the surface of the Earth. It’s a natural process that recycles water and is essential for life.
Stages of the Water Cycle
Evaporation:
– Description: Water from oceans, lakes, and rivers is heated by the sun and changes into water vapor.
– Example: The steam you see from boiling water is an example of evaporation.
Condensation:
– Description: Water vapor rises into the air and cools down, turning back into tiny water droplets, forming clouds.
– Example: Clouds in the sky are formed from condensed water vapor.
Precipitation:
– Description: When the clouds become heavy with water droplets, they release the water as rain, snow, sleet, or hail.
– Example: Rain falling from the sky is a result of precipitation.
Collection:
– Description: The precipitation collects in rivers, lakes, and oceans. Some of it also soaks into the ground, replenishing groundwater.
– Example: Rainwater flowing into a river or soaking into the soil.
Runoff:
– Description: Water from precipitation flows over the ground and returns to larger bodies of water like lakes, rivers, and oceans.
– Example: Water flowing down a hill and into a river.
The water cycle is essential for replenishing our water sources and supporting life on Earth. It ensures that water is always moving and available in different forms.
Properties of Water
Water has several unique properties that make it essential for life. Let’s explore some of these properties:
Universal Solvent
– Description: Water can dissolve many substances, which makes it an excellent solvent.
– Examples: Sugar dissolving in tea, salt dissolving in seawater.
High Specific Heat
– Description: Water can absorb and release a lot of heat without changing temperature significantly. This property helps regulate temperatures in living organisms and the environment.
– Examples: Coastal areas have milder temperatures because water absorbs heat in the summer and releases it in the winter.
Adhesion and Cohesion
– Description: Water molecules stick to other substances (adhesion) and to themselves (cohesion). This property helps water move through plants and creates surface tension.
– Examples: Water climbing up a plant’s stem, water droplets forming on a surface.
Expands When Frozen
– Description: Unlike most substances, water expands when it freezes, making ice less dense than liquid water.
– Examples: Ice floats on water, which is important for aquatic life in frozen lakes.
Transparency
– Description: Water is clear, allowing sunlight to penetrate and support aquatic plant life.
– Examples: Light reaching underwater plants in lakes and oceans.
Importance of Water
Water is vital for all forms of life and has many important uses. Let’s look at why water is so essential:
Supporting Life
– Plants: Water is essential for photosynthesis, the process by which plants make their food. It also helps plants absorb nutrients from the soil.
– Animals: All animals need water to drink, regulate their body temperature, and perform various bodily functions.
– Humans: Humans need water for drinking, cooking, cleaning, and maintaining health. Water also helps in digestion and nutrient absorption.
Agriculture
– Description: Water is crucial for growing crops and raising livestock. Without sufficient water, crops would not survive, and food supplies would be at risk.
– Example: Irrigation systems use water to keep fields and farms productive.
Industry
– Description: Many industrial processes require water, such as manufacturing, cooling, and cleaning.
– Example: Water is used in the production of goods, cooling machinery, and as a solvent in chemical processes.
Recreation
– Description: Water provides enjoyment and relaxation in activities like swimming, boating, and fishing.
– Example: Beaches, lakes, and swimming pools are popular recreational spots.
Climate Regulation
– Description: Water helps regulate the Earth’s climate by absorbing and releasing heat. It also influences weather patterns.
– Example: Oceans and large lakes can moderate temperatures and affect local weather.
How to Store Drinking Water
Proper storage of drinking water is important to ensure it remains clean and safe. Here are some tips for storing drinking water:
Use Clean Containers
– Description: Always use clean, food-grade containers for storing water. Avoid using containers that have previously held chemicals or non-food substances.
– Example: Plastic or glass bottles labeled for food use.
Store in a Cool, Dark Place
– Description: Keep water stored in a cool, dark place to prevent algae growth and maintain its quality.
– Example: Store water bottles in a cupboard or a basement rather than in direct sunlight.
Keep Lids Tight
– Description: Ensure that containers are tightly sealed to prevent contamination from dust, dirt, or insects.
– Example: Always close bottle caps or container lids securely after use.
Rotate Stock
– Description: Use and replace stored water regularly to ensure it remains fresh.
– Example: Use the oldest water first and replace it with fresh supplies.
Check for Contamination
– Description: Regularly check stored water for any signs of contamination, such as unusual smells or colors.
– Example: Discard any water that appears cloudy or has a strange odor.
The worksheet covers the following topics-
Forms of Water
Solid Form- Ice
Liquid Form- Water
Gaseous Form- Water vapor
Water cycle
Properties of water
Importance of water
How to store drinking water